How To: Use the TMF Reference Model
The TMF Reference Model is an industry-adopted reference TMF structure that takes the form of an index. Think of the TMF Reference Model like the taxonomy that biologists use to classify all the various types of life on Earth. Or consider another familiar index, the Dewey Decimal System which helps keep books organized in a library. Instead of helping organize species of beetles or paperbacks in a useful and consistent way, the TMF Reference Model helps clinical researchers organize the documents in their TMF.
The TMF Reference Model is not required by regulation but rather began as a long-running project of the Document and Records Management Community of the DIA, the Drug Information Association. The DIA is a more than fifty-year-old global industry association of companies and individuals who contribute to healthcare product development and management. The wide industry adoption of the TMF Reference Model is a testament to the value it has provided—even as a free tool made by volunteers. No single individual contributor, company, or organization owns the TMF Reference Model. It is published in the public domain and is free for use by anyone for any purpose without restriction.
Recently, the TMF Reference Model Working Group of the DIA announced that the TMF Reference Model Group has officially affiliated with CDISC. While our blog on the subject covers the importance of this affiliation in detail, it’s essential for TMF professionals to know that affiliation with CDISC, or the Clinical Data Interchange Standards Consortium, a standards development organization, is a significant step toward having the TMF recognized as an official standard. As an official standard, the TMF Reference Model could one day be recognized formally by the FDA and other regulatory agencies. In other words, you can feel confident that the TMF Reference Model’s place in the industry will only continue to grow.
How: How do you use the Reference Model?
Version 3.2.1, the current version of the TMF Reference Model, is presented here within an Excel spreadsheet. The link to the most current version of the TMF Reference model is also located in the upper right-hand corner of the TMF Reference Model website. We encourage you to open the link to download the spreadsheet and follow along as we describe the basic features of the TMF Reference Model.

Screenshot of the TMF Reference Model Home Page with link to the current version (Version 3.2.1) of the TMF Reference Model.
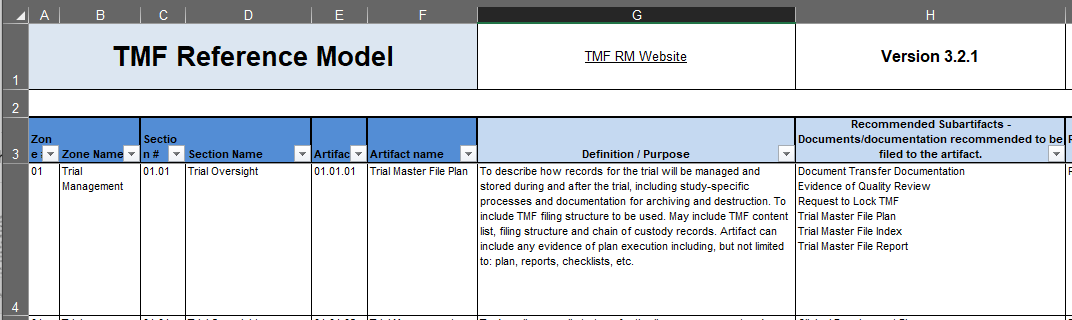
First opening the TMF Reference Model can be overwhelming because of the sheer amount of information within the spreadsheet. Those unfamiliar with the TMF Reference Model should navigate to the first tab titled “Ver 3.2.1 Clean”. This tab is the ‘home page’ of the TMF Reference Model and contains the full document taxonomy. As you become more familiar with using the TMF Reference Model to assist you, you’ll find that you use this tab most of the time.
Focus on columns A-H. The TMF Reference Model attempts to sort every relevant clinical trial document into a pre-defined location. The incoming document is first compared against a limited number of high-level Zones (columns A and B) that account for coarse distinctions between certain documents: Did the document come from data management? Does the document relate to an Ethics Committee? Is it related to the investigational product handling? For example, let’s say the document you want to file is an IRB approval—so it would be filed somewhere within Zone 04 (IRB and other Approvals).
Within each Zone, there are Sections, Artifacts, and Sub-Artifacts. Sections (columns C and D) provide greater granularity within a Zone: Is the IRB document, referenced above, related to a submission or an approval? Is it just an email about the IRB? An Artifact (columns E and F) represents a type of document, like Meeting Materials or Safety Notifications. Finally, within each Artifact are variants on that Artifact, known as Subartifacts (column H): Is the IRB approval conditional or full? Is this document only an acknowledgement of receipt?
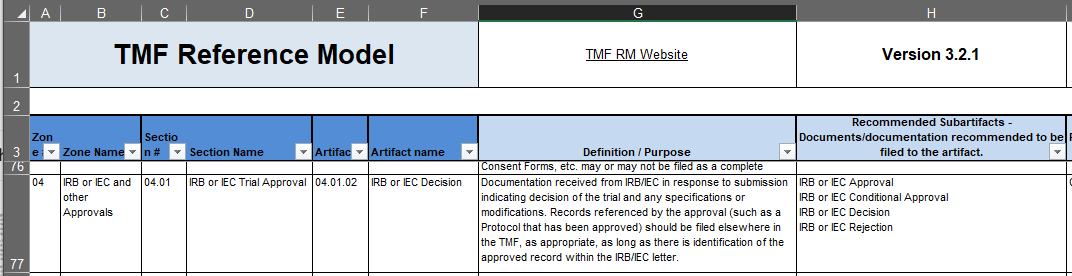
As your document moves from the least specific Zones to the most specific Artifacts or Subartifacts, ultimately finding a filing location, it will accumulate the numbers listed in columns A, C, and E. Our example document, an IRB approval, would be filed in 04.01.02, IRB or IEC Approval. We can confirm that we have the right artifact by reading the definition/purpose for artifact 04.01.02 in column G, row 76, “Documents describing the trial or changes/updates to the trial submitted to an IRB/IEC for approval, including recruitment and education materials and responses to questions from IRB/IEC to support a submission”.
Once you are familiar with the basic structure, you can begin to move right on the spreadsheet to consider more advanced concepts like what TMF Level a document belongs, what date convention is used for the document, and what metadata would be expected for a specific artifact. Yes, the Reference Model is a sophisticated tool, but the basics aren’t any more complicated the putting a book on the shelf in a library.
Why: Why is the Reference Model important?
To appreciate why the Reference Model is important, consider what it was like before the Reference Model was used, when every life sciences company had their own TMF structure with unique document naming and metadata. This huge variability in TMF structure, at least in part, was the product of vague regulation. After all, the list of essential documents required by name in regulation is only a small portion of what is required for an inspection ready TMF, and the list itself could be out of date only a few years after the last update. Imprecise regulation, in conjunction with rapidly advancing science and ever-increasing clinical trial complexity, meant in the past, without a standardized TMF index, TMF content and structure was doomed to be fragmented across industry.
Unsurprisingly, the inconsistency between TMFs spawned significant real-world challenges. For example, when TMF stakeholders changed companies (or even studies), they usually had to learn a new TMF structure from scratch. Worse yet, when TMFs changed hands with mergers and acquisitions, chaos ensued. The risks associated with TMF management were growing untenable and led to groups like the Document and Records Management Community of the DIA laying the foundation for the TMF Reference Model we depend on today.
In this way, the TMF Reference Model, by ensuring that documents can easily be compared, moved, and grouped based on common attributes, truly constitutes a significant advancement in clinical operations and clinical research as whole. Although the Reference Model is not a perfect one-size-fits-all solution, it has greatly paved the way for the life sciences industry to finally realize the full transformative potential of the eTMF and related digital document and data management technology.