Three Key Conclusions: New MHRA GCP Inspection Metrics
Great Britain’s MHRA (Medicines and Healthcare products Regulatory Agency) is known (and feared) for their high expectations regarding the TMF. MHRA expects your TMF to be “the story of how the trial was conducted and managed.” Their “inspectors want to reconstruct the trial conduct so that it can be evaluated for compliance …[and] review any documentation, data and metadata required…”
Given MHRA’s expectations, it is no surprise that the TMF is well represented among the findings in their recently released GCP Inspections Metrics Report for April 2016 through March 2017. Although it is too late for those who have already been inspected, MHRA’s report provides key insights that can be incorporated to contribute to your own inspection readiness. We’ve reviewed the findings and summarized what we’ve identified as this year’s most critical TMF conclusions:
1. Findings are Assured
“A total of 16 commercial sponsors were inspected… 7 (46.7%) had at least one critical finding and all (100 %) had at least one major and/or critical finding”.

Regarding CROs: “Of the 8 inspections, 2 (25.0 %) had critical findings and 7 (87.5%) had at least one major finding. The total number of findings and the findings per inspection are represented on the figure(s) below.”
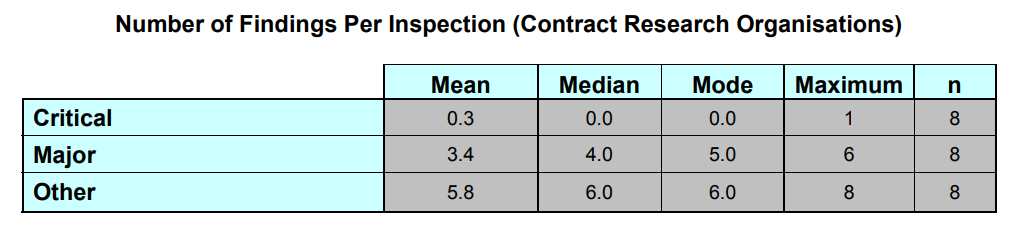
The tables (excerpts from the report) show that both CROs and Sponsors do not usually receive critical findings. Most sponsors, however, received two or three major findings and six or seven other less consequential findings. CROs, fared slightly better with one fewer finding on average, and lower mean issuance of critical findings. The most striking metric, however, shows that all inspected Sponsors were issued at least one major or critical finding.
2. TMF Related Finding Rates are Not Decreasing for Both Sponsors and CROs
The following graph excerpts from this year’s inspection report show the percentage distribution of findings for the three categories (purple meaning total findings, green being major/other grading, and red meaning major grading). Items labeled 2015-2016 are extracted from the 2015-2016 GCP Inspection Metrics Report. Items labeled 2016-2017 are extracted from the current inspection report.
CRO 2015-2016 Inspections (the horizontal grey bars correspond with five percent increments beginning at 0%)

Approximately 14% of major findings and 11% of all findings. TMF related findings represent the second greatest percentile allocation of major findings.
CRO 2016-2017 Inspections (the horizontal grey bars correspond with two percent increments beginning at 0%)
![]()
Approximately 15% of major findings and 10% of all findings. TMF related findings represent the greatest percentile allocation of major findings and of all findings.
Commercial Sponsor 2015-2016 Inspections (the horizontal grey bars correspond with two percent increments beginning at 0%)

Approximately 17% of major findings and 9% of all findings. TMF related findings represent the single greatest percentage allocation for all findings.
Commercial Sponsor 2016-2017 Inspections (the horizontal grey bars correspond with five percent increments beginning at 0%)

Approximately 15% of major findings and 8 % of all findings. TMF related findings represent the second greatest allocation of major findings.
From this data it is easy to draw the conclusion that the increased guidance provided by MHRA and the increase of adoption of eTMF technology have not succeed in reducing the total number of TMF related findings (and thus may not have sufficiently balanced MHRA’s increased TMF expectations).
3. Critical Findings are Frequently TMF Related
As part of each MHRA GCP inspection report, summaries of major findings are included. The summaries of these major findings lend credence to the idea that regardless of whether a major finding is labeled as relating to ‘essential documents’, usually the TMF plays a role in the noncompliance. Of the twelve critical findings listed for CROs and Commercial Sponsors, three were directly issued for ‘Record Keeping/Essential Documents’:
Finding 3 (Commercial Sponsor): “The TMF was presented as a paper TMF for inspection. However, the TMF did not contain all the essential documents required to enable the reconstruction of trial events and demonstrate compliance with the regulations and the organisation’s own quality system.”
Finding 8 (Commercial Sponsor): “The inspector requested documents that could not be located in the eTMF. Despite the assistance of the study team and the eTMF experts, not all these documents could be found over the 4-day inspection, and those that were provided took two days to locate.”
Finding 2 (CRO): “The inspection was extended as it required the Inspectors to return after four months to enable review of clinical trial compliance…It took the full four months to ensure the completeness of the three selected TMFs. Over 3000 documents had been created/uploaded in to one of the trial TMFs and over 5000 documents had been uploaded in to another of the selected TMFs.”
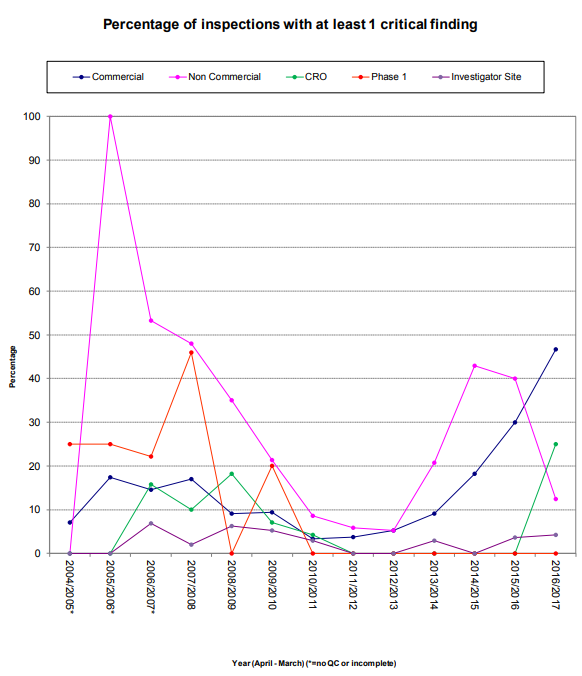 It is also important to note that of the twelve critical findings, five referenced the inability to locate documentation of a specific activity within the TMF as evidence of the noncompliance. Additionally, both instances of serious TMF related findings also resulted in pharmacovigilance findings because the general failure of the TMF meant, “significant potential for Suspected Unexpected Serious Adverse Reactions (SUSARs) to go unreported.”
It is also important to note that of the twelve critical findings, five referenced the inability to locate documentation of a specific activity within the TMF as evidence of the noncompliance. Additionally, both instances of serious TMF related findings also resulted in pharmacovigilance findings because the general failure of the TMF meant, “significant potential for Suspected Unexpected Serious Adverse Reactions (SUSARs) to go unreported.”
To Summarize
Overall the data “shows a general improvement following the introduction of statutory GCP inspections”, but this trend has not necessarily extended to the Sponsor and CRO. Regardless of the circumstances of the inspection or type of establishment, however, it is clear MHRA sees the TMF as the window into the clinical trial that should not be a final document repository, but “a contemporaneous system used to manage the trial.” MHRA’s focus on the TMF is not simply meant to place a greater burden on those who conduct clinical research but stems from a clear truth: a healthy TMF is the basis of a healthy and compliant trial.
https://mhrainspectorate.blog.gov.uk/2015/07/30/inspecting-clinical-trials-the-trial-master-file/

